Lupine Publishers Journal of Surgery and Journal of Case Studies: Currently case studies drag the concentration of the investigators since each case present provides deep understanding in diagnosis and treatment methods. It is devoted to publishing case series and case reports. Articles must be genuine
Thursday, October 21, 2021
Lupine Publishers: Lupine Publishers| Reduced Graphene Oxide Via Gree...
Monday, October 18, 2021
Lupine Publishers: Lupine Publishers | The Effect of Some Natural Fer...
Friday, October 8, 2021
Lupine Publishers|Antioxidant and Organ Protective Potential of the Consciousness Energy Healing-Based Novel Test Formulation Using Different Biomarkers Analysis
Abstract
The aim of this paper was to investigate the impact of the Biofield Energy Treatment on the test formulation by focusing on the function of vital organs viz. bones, heart, liver, lungs, and brain in various cell-based assays. The test formulation and the cell media was divided into two parts; one part was untreated (UT) and other part received the Biofield Energy Treatment remotely by a renowned Biofield Energy Healer, Kathryn Regina Sweas, USA and was labeled as the Biofield Energy Treated (BT) test formulation/ media. Cell viability data suggested that the test formulation was safe and non-toxic in nature in six different cells. The experimental group of Biofield Treated medium (BT-Med) + Biofield Treated Test Item (BT-TI) group showed 53.5%, 127.9%, and 53.3% restoration of cell viability, at 0.1, 10, and 25 µg/mL, respectively in human cardiac fibroblasts cells (HCF) compared to the UTMed + UT-TI group. Moreover, UT-Med + BT-TI and BT-Med + BT-TI groups showed 42.4% (at 25 µg/mL) and 72.6% (at 0.1 µg/mL), restoration of cell viability, respectively in human hepatoma cells (HepG2) compared to untreated. Furthermore, 67.7% restoration of cell viability was observed in adenocarcinomic human alveolar basal epithelial cells (A549) by BT-Med + BT-TI group at 0.1 µg/ mL compared to the untreated. The alkaline phosphatase (ALP) level was significantly increased by 80.7%, 85%, and 93.7% in the UT-Med + BT-TI, BT-Med + UT-TI, and BT-Med + BT-TI groups, respectively at 10 µg/mL in human bone osteosarcoma cells (MG-63) compared to the untreated. Additionally, the level of ALP was significantly increased by 65.8% (at 25 µg/mL) and 106.7% (at 50 µg/mL) in the BT-Med + UT-TI and BT-Med + BT-TI groups, respectively in human endometrial adenocarcinoma cells (Ishikawa) compared to the untreated. The percent protection of HCF (heart) cells (decreased of LDH activity) was significantly increased by 100.9% (at 0.1 µg/mL) and 91.2% (at 10 µg/mL) in the UT-Med + BT-TI and BT-Med + BT-TI groups, respectively compared to the untreated in HCF cells. The percent protection of HepG2 (liver) cells (decreased of ALT activity) was significantly increased by 51.5% and 133.6% at 10 and 63 µg/mL, respectively in the BT-Med + BT-TI group compared to untreated in HepG2 cells. The percent protection of A549 (lungs) cells (increased of SOD activity) was significantly increased by 55.8% (at 10 µg/mL) and 43.8% (at 1 µg/ mL) in the UT-Med + BT-TI and BT-Med + UT-TI groups, respectively compared to the untreated in A549 cells. Serotonin level was significantly increased by 80%, 77.2%, and 58.7% in the BT-Med + BT-TI group at 10, 25, and 63 µg/mL, respectively as compared to untreated in human neuroblastoma cells (SH-SY5Y). The relative quantification (RQ) of vitamin D receptor (VDR) was significantly increased by 136.8%, 191.9%, and 165.8% in the UT-Med + BT-TI, BT-Med + UT-TI, and BT-Med + BT-TI groups, respectively at 50 µg/mL compared to the untreated in MG-63 cells. Altogether, results suggest that Biofield Treated test formulation significantly improved the bones, heart, liver, lungs, and brain functional enzyme biomarkers also to protect and maintain the normal function of each vital organs. Therefore, The Trivedi Effect® can be used as a complementary and alternative therapy against several disorders such as coronary artery disease, heart attack, congenital heart disease, heart failure, arrhythmias, cirrhosis, cardiomyopathy, liver cancer, Wilson disease, hemochromatosis, pneumonia, chronic bronchitis, asthma, cystic fibrosis, emphysema, osteoporosis, etc.
Keywords: The Trivedi Effect®; Consciousness Energy Treatment, Bone health; Liver health; Cardiac health; Lungs health; Brain health; VDR receptor
Introduction
Bones, heart, liver, lungs, and brain disorders are the major concern of human overall health across the globe. The World Health Organization (WHO) estimates, in 2016, ~17.5 million people die due to cardiovascular (heart) disorders, ~3.5 million people die due to lungs disorders, ~1.3 million people die due to liver disorders around the globe each year [1]. Moreover, ~1.2 million people most frequently diagnosed adult-onset brain disorders in each year in the USA [2]. Three main criteria to keep a healthy heart include the opening blood vessels, strengthening the heart muscle, and controlling free radical damage by antioxidants [3]. The release of liver mitochondrial enzymes is considered strong evidence for hepatic (liver) necrosis, which is associated with an increased production of reactive oxygen species (ROS) that leads to hepatic lipid peroxidation [4-6]. Oxidative stress in the respiratory system increases the production of mediators of pulmonary inflammation and initiate or promote mechanisms of carcinogenesis [7]. The lung is one of the major organs, which is highly exposed by various oxidants i.e., endogenous and exogenous oxidants (cigarette smoke, mineral dust, ozone, and radiation). These oxidants produce free radicals, while reactive oxygen species (ROS) and reactive nitrogen species (RNS) are produced by phagocytes as well as by alveolar, polymorphonuclear, bronchial and different endothelial cells [8]. However, the role of oxidative stress in the pathogenesis of lung diseases has been widely reported such as asthma, chronic obstructive pulmonary disease (COPD), lung malignancies and parenchymal lung diseases like idiopathic pulmonary fibrosis and lung granulomatous diseases [9]. Serotonin (5-hydroxytryptamine, 5-HT) is among the brain’s neuromodulators responsible for behavior and understanding [10]. Apart from medicines, nonpharmacologic methods that can increase serotonin by increasing recognition and happiness and well-being. These factors can protect against mental and physical disorders [11]. There is currently no universally accepted test formulation, which improve the organ health biomarkers. With this respect, the novel test formulation was designed on the basis of best scientific literature, which is the combination of herbal products viz. panax ginseng extract and beta carotene, minerals viz. calcium chloride, magnesium gluconate, zinc chloride, sodium selenate, ferrous sulfate, and vitamins viz. vitamin B12, vitamin D3 , ascorbic acid, and vitamin B6 . This formulation is designed for overall functioning of the organs that can results in improved overall health conditions against many pathological conditions such as lung disorder, liver disorder, breast cancer, liver cancer, aging, muscle damage, and overall health. Minerals and vitamins present in the test formulation provide significant functional support to all the vital organs [12-14]. In addition, panax ginseng is one of the best reported medicinal plants that improve mental, physical abilities, cognitive health, and is potent immunomodulator [15,16].
Various study data suggested the effect of Energy Therapy in cancer patients through therapeutic touch [17], massage therapy [18], etc. Complementary and Alternative Medicine (CAM) therapies are preferred model of treatment, among which Biofield Therapy (or Healing Modalities) is one approach to enhance emotional, mental, physical, and human wellness. The National Center of Complementary and Integrative Health (NCCIH) has recognized and allowed Biofield Energy Healing as a CAM approach in addition to other therapies and medicines such as natural products, chiropractic/osteopathic manipulation, Qi Gong, deep breathing, Tai Chi, yoga, meditation, massage, special diets, healing touch, relaxation techniques, traditional Chinese herbs and medicines, naturopathy, movement therapy, homeopathy, progressive relaxation, guided imagery, pilates, acupuncture, acupressure, Reiki, rolfing structural integration, hypnotherapy, Ayurvedic medicine, mindfulness, essential oils, aromatherapy, and cranial sacral therapy. The Human Biofield Energy has subtle energy that has the capacity to work in an effective manner [19]. CAM therapies have been practiced worldwide with reported clinical benefits in different health disease profiles [20]. This energy can be harnessed and transmitted by the practitioners into living and non-living things via the process of Biofield Energy Healing. The Biofield Energy Treatment, the Trivedi Effect®, has been reported to have a significant impact in the field of cancer research [21,22], materials science [23,24], microbiology [25,26], agriculture [27,28], nutraceuticals [29,30], and biotechnology [31, 32]. Further, the Trivedi Effect® also significantly improved bioavailability of various low bioavailable compounds [33-35], an improved overall skin health [36,37], bone health [38-40], human health and wellness. Based on the excellent outcomes of the Biofield Energy Therapy in wide spectrum of areas, the authors intend to see the impact of the Biofield Energy Healing Treated test formulation on the function of vital organs such as bones, heart, liver, lungs, and brain specific biomarkers in different cell-lines.
Materials and Methods
Chemicals and Reagents
Ferrous sulfate, vitamin B6 , vitamin D3 , vitamin B12, calcium chloride, naringenin, trimetazidine (TMZ), 3-(4,5-Dimethylthiazol2-yl)-2,5-Diphenyltetrazolium Bromide (MTT), and ethylenediaminetetraacetic acid (EDTA) were obtained from Sigma Chemical Co. (St. Louis, MO). Zinc chloride, magnesium gluconate, β-carotene, and calcitriol were purchased from TCI chemicals, Japan. Panax ginseng extract obtained from panacea Phytoextracts, India. Sodium selenate and ascorbic acid were obtained from Alfa Aesar, India. Silymarin and curcumin were obtained from Sanat Chemicals, India and quercetin obtained from Clearsynth, India. Reverse Transcription Kit, RNeasy Mini Kit, and Syber Green PCR kits were procured from Quagen, India. All the other chemicals used in this experiment were analytical grade procured from India.
Biofield Energy Healing Strategy
The test formulation was the combination of eleven ingredients viz. calcium chloride, panax ginseng extract, vitamin B12, β-carotene, vitamin D3 , zinc chloride, magnesium gluconate, sodium selenate, ferrous sulfate, ascorbic acid, and vitamin B6 . The test formulation and the cell media was divided into two parts; one untreated (UT) and other part received the Biofield Energy Treatment remotely by a renowned Biofield Energy Healer, Kathryn Regina Sweas under laboratory conditions for ~3 minutes through healer’s unique Biofield Energy Transmission and was labeled as the Biofield Energy Treated (BT) test formulation/media. Further, the untreated group was treated with a “sham” healer for comparison purposes. The “sham” healer did not have any knowledge about the Biofield Energy Healing Treatment. The Biofield Energy Healer was located in the USA however the test items were located in the research laboratory of Dabur Research Foundation, New Delhi, India. Biofield Energy Healer in this experiment did not visit the laboratory, nor had any contact with the test samples. After that, the Biofield Energy Treated and untreated test items were kept in similar sealed conditions and used for the study as per the study plan.
Assessment of Cell Viability using MTT Assay
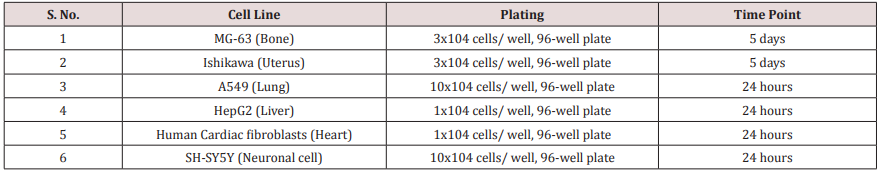
Cells were counted using hemocytometer and plated in 96- well plates at the specific density described in (Table 1). The cells were then incubated overnight under growth conditions to allow cell recovery and exponential growth. Following overnight incubation, cells were treated with different concentrations of test formulations (BT/UT). Following respective treatments, cells were incubated in a CO2 incubator at 37°C, 5% CO2 , and 95% humidity and incubated for time period mentioned in (Table 1). After incubation, the plates were taken out and 20 µL of 5 mg/ mL of MTT 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide solution was added to all the wells followed by additional incubation for 3 hours at 37°C. The supernatant was aspirated and 150 µL of DMSO was added to each well to dissolve formazan crystals. The absorbance of each well was read at 540 nm using Synergy HT microplate reader. The percentage cytotoxicity at each tested concentration of TI was calculated using Equation 1:
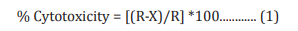
Where, X = Absorbance of treated cells; R = Absorbance of untreated cells
The concentrations exhibiting percentage cytotoxicity < 30% were considered as non-cytotoxic [41].
Evaluation of the cytoprotective effect of the formulation
Cells (human cardiac fibroblasts-HCF; human hepatoma cellsHepG2; and adenocarcinomic human alveolar basal epithelial cells-A549) were counted and plated in suitable medium followed by overnight incubation. The cells were then treated with the test items/positive control at the non-cytotoxic concentrations for 24 hours. After 24 hours, oxidative stress was given to the cells using 10 mM t-BHP for 3.5 hours. The untreated cells served as a control that did not receive any treatment and was maintained in cell growth medium only. Cells treated with 10 mM of t-BHP alone served as negative control. After 3.5 hours of incubation with t-BHP the above plates were taken out and cell viability was determined by MTT assay. The percentage protection corresponding to each treatment was calculated using Equation 2:
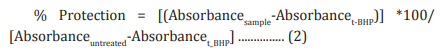
Assessment of alkaline phosphatase (ALP) activity The cells (human bone osteosarcoma cells-MG-63 and human endometrial adenocarcinoma cells-Ishikawa) were counted using a hemocytometer and plated in 24-well plates at the density corresponding to 1 X 104 cells/well in phenol-free DMEM supplemented with 10% CD-FBS. Following the respective treatments, the cells in the above plate were incubated for 48 hours in CO2 incubator at 37°C, 5% CO2 , and 95% humidity. After 48 hours of incubation, the plates were taken out and processed for the measurement of ALP enzyme activity. The cells were washed with 1 X PBS and lysed by freeze-thaw method i.e., incubation at -80°C for 20 minutes followed by incubation at 37°C for 10 minutes. To the lysed cells, 50 µL of substrate solution i.e., 5 mM of p-nitrophenyl phosphate (pNPP) in 1M diethanolamine and 0.24 mM magnesium chloride (MgCl2 ) solution (pH 10.4) was added to all the wells followed by incubation for 1 hour at 37°C. The absorbance of the above solution was read at 405 nm using Synergy HT microplate reader (Biotek, USA). The absorbance values obtained were normalized with substrate blank (pNPP solution alone) absorbance values. The percentage increase in ALP enzyme activity with respect to the untreated cells (baseline group) was calculated using Equation 3:
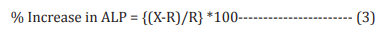
Where, X = Absorbance of cells corresponding to positive control and test groups
R = Absorbance of cells corresponding to baseline group (untreated cells)
Estimation of lactate dehydrogenase (LDH) in human cardiac fibroblasts (HCF)
The human cardiac fibroblasts (HCF) Cells were counted and plated at the density of 0.25 X 106 cells/ well in 24-well plates in cardiac fibroblast specific medium followed by overnight incubation. The cells were then treated with the test formulation/positive control at the non-cytotoxic concentrations for 24 hours. After 24 hours, oxidative stress was given to the cells using 10 mM t-BHP for 3.5 hours. The untreated cells were served as control that did not receive any treatment and were maintained in cell growth medium only. Cells treated with 10 mM of t-BHP alone served as the negative control. After 3.5 hours of incubation with t-BHP the above plates were taken out and LDH activity was determined using LDH activity kit as per manufacturer’s instructions. The percent increase in LDH activity was calculated using Equation 4.
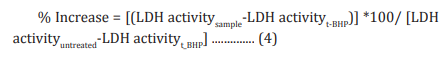
Estimation of ALT in liver cells (HepG2)
The human hepatoma cells (HepG2) were counted and plated at the density of 5 X 104 cells/well in 48-well plates in DMEM media followed by overnight incubation. The cells were then treated with the test formulation/positive control at the non-cytotoxic concentrations for 24 hours. After 24 hours, oxidative stress was given to the cells using 400 µM t-BHP for 3.5 hours. The untreated cells served as control that did not receive any treatment and were maintained in cell growth medium only. Cells treated with 400 µM of t-BHP alone served as negative control. After 3.5 hours of incubation with t-BHP the above plates were taken out and ALT activity was determined using ALT activity kit as per manufacturer’s instructions. The percent increase in ALT activity was calculated using Equation 5.
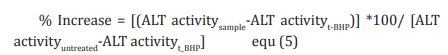
Estimation of superoxide dismutase (SOD) in lung (A549) cells
The adenocarcinomic human alveolar basal epithelial cells (A549) were counted and plated at the density of 1 X 104 cells/well in 24-well plates in DMEM followed by overnight incubation. The cells were then treated with the test formulation/ positive control at the non-cytotoxic concentrations along with 100 µM t-BHP to induce oxidative stress. The untreated cells served as control that did not receive any treatment and were maintained in cell growth medium only. Cells treated with 100 µM of t-BHP alone served as negative control. After 24 hours of incubation with t-BHP the above plates were taken out and SOD activity was determined using SOD activity kit as per manufacturer’s instructions. The percent increase in SOD activity was calculated using Equation 6:
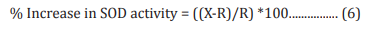
Where, X = SOD activity corresponding to Test Item or Positive Control
R = SOD activity corresponding to Control group.
Estimation of serotonin in neuronal cells (SH-SY5Y)
The human neuroblastoma (SH-SY5Y) cells were counted and plated at the density of 10 X 104 cells/well in 96-well plates followed by overnight incubation. The cells were then treated with the test items/positive control at the non-cytotoxic concentrations. The untreated cells served as control that did not receive any treatment and were maintained in cell growth medium only. The treated cells were incubated for 24 hours. Serotonin release was determined by ELISA as per manufacturer’s protocol. The percent increase in serotonin levels was calculated using Equation 7.
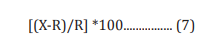
Where, X = Serotonin levels corresponding to test item or positive control
R = Serotonin levels corresponding to control group.
Effect of test formulation on vitamin D receptor (VDR) in bone (MG-63) cells
The human bone osteosarcoma (MG-63) cells were counted using the hemocytometer were plated at a density of 2 X 105 cells/ well in 6-well plates followed by overnight incubation. The cells were then sera starved for 24 hours and treated with the test formulation/positive control at the non-cytotoxic concentrations. The untreated cells that served as control that did not receive any treatment and were maintained in cell growth medium only. The treated cells were incubated for 24 hours and VDR expression was determined by Q-PCR using VDR specific primers. Cells were harvested by scrapping and washed with PBS. Cell pellets obtained were analyzed for VDR gene expression using human VDR specific primers: Forward: 5’-GCTGACCTGGTCAGTTACAGCA-3’, Reverse: 5’-CACGTCACTGACGCGGTACTT-3’. VDR gene expression was normalized using House-keeping (HK) reference. Relative quantification (RQ) of VDR gene in Biofield Energy Treated cells was calculated with respect to the untreated cells using Equation 8:

Where N is the relative Threshold Cycle (CT) value of treated sample with respect to the untreated sample.
Statistical analysis
All the values were represented as Mean ± SD (standard deviation) of three independent experiments. The statistical analysis was performed using Sigma Plot statistical software (v11.0). For two groups comparison student’s t-test was used. For multiple group comparison, one-way analysis of variance (ANOVA) was used followed by post-hoc analysis by Dunnett’s test. Statistically significant values were set at the level of p≤0.05.
Results and Discussion
Cell viability using MTT assay
Determination of non-cytotoxic concentration of the formulation and positive controls by MTT cell viability assay was used in terms of percent viable cells in six (6) different cell-lines viz. MG-63, Ishikawa, A549, HepG2, HCF, and SH-SY5Y. Based on the percent cell viability data, it was observed that the formulation and positive controls were safe and non-toxic at the tested concentrations in six different cell lines and selected for other parameters analysis.
Evaluation of cytoprotective effect of the test formulation
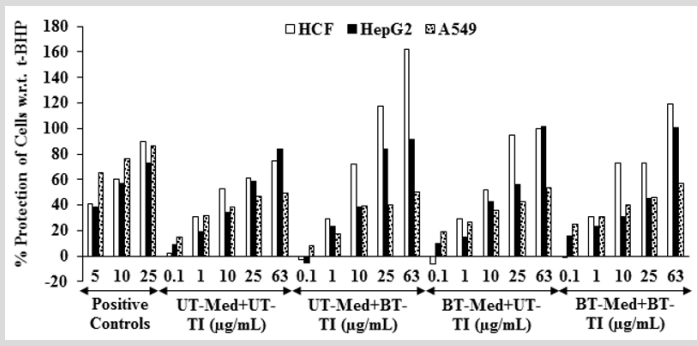
The cytoprotective activity of the Biofield Energy Treated test formulation on the restoration of cell viability was determined against t-BHP induced cell damage and the result is shown in Figure 1. Trimetazidine (TMZ) was used as positive control in human cardiac fibroblasts cells (HCF) and showed, restoration of cell viability by 56.61%, 94.09%, and 102.29% at 5, 10, and 25 µg/ mL, respectively compared to the t-BHP induced group. Besides, the test formulation showed 53.5% restoration of cell viability at 0.1 µg/mL in the BT-Med + BT-TI group as compared to the UT-Med + UT-TI group. Moreover, at 1 µg/mL the UT-Med + BT-TI and BTMed + UT-TI group showed 34.1% and 12.9% restoration of cell viability, respectively than UT-Med + UT-TI group. Additionally, the test formulation showed 23.7% and 127.9% restoration of cell viability at 10 µg/mL in the BT-Med + UT-TI and BT-Med + BT-TI groups, respectively as compared to the UT-Med + UT-TI group. Further, at 25 µg/mL the test formulation showed 52.9% and 53.3% restoration of cell viability, respectively in the BT-Med + UT-TI and BT-Med + BT-TI groups, respectively than UT-Med + UTTI group. Further, the test formulation showed 33.1% and 35.1% restoration of cell viability at 63 µg/mL in the UT-Med + BT-TI and BT-Med + BT-TI groups, respectively as compared to the UT-Med + UT-TI group (Figure 1). Silymarin was used as positive control in human hepatoma cells (HepG2) resulted, restoration of cell viability by 38.4%, 56.6%, and 72.6% at 5, 10 and 25 µg/mL, respectively compared to the t-BHP induced group. Besides, the test formulation showed 42.4% (at 25 µg/mL), 23.0% (at 10 µg/mL), and 72.6% (at 0.1 µg/mL) restoration of cell viability in the UT-Med + BT-TI, BTMed + UT-TI, and BT-Med + BT-TI groups, respectively as compared to the UT-Med + UT-TI group (Figure 1). Quercetin was used as positive control in adenocarcinomic human alveolar basal epithelial cells (A549) resulted, restoration of cell viability by 65.7%, 76.7%, and 86.1% at 5, 10 and 25 µg/mL, respectively compared to the t-BHP induced group. Besides, the test formulation showed 28.2% and 67.7% restoration of cell viability at 0.1 µg/mL in the BT-Med + UT-TI and BT-Med + BT-TI groups, respectively compared to the UT-Med + UT-TI group (Figure 1). Tert-butyl hydroperoxide (t-BHP) has been extensively utilized as an oxidative stress marker in various cells [41]. In this study, t-BHP was used an oxidative stress inducer for the assessment of various vital organs viz. heart, liver, and lungs using cell-based assay. Thus, restoration of cell viability from t-BHP induced oxidative stress by Biofield Energy Treated novel proprietary test formulation could be due to the either upregulated the expression of heme oxygenase 1 (HO-1) and NAD(P) H quinone oxidoreductase 1 (NQO1) or induction of antioxidantresponsive-element (ARE)-dependent luciferase activation, nuclear factor (erythroid-derived 2)-like 2 (Nrf2) nuclear translocation, and mitogen-activated-protein-kinase (MAPK) phosphorylation [42]. The study results suggest that Biofield Energy Treatment has significantly protects t-BHP induced cardiotoxicity, hepatotoxicity, and lung cell toxicity which could be due to The Trivedi Effect®- Biofield Energy Healing. Therefore, Biofield Energy Healing Treatment could be used for the management of cardiovascular, liver, and various lung disorders.
Figure 1: Assessment of cytoprotective effect of the test formulation in human cardiac fibroblasts cells (HCF), human hepatoma cells (HepG2), and adenocarcinomic human alveolar basal epithelial cells (A549) against tert-butyl hydroperoxide (t-BHP) induced damage. TMZ: Trimetazidine (µM), silymarin (µg/mL), and quercetin (µM) were used as positive control in HCF, HepG2, and A549 cells, respectively. UT: Untreated; Med: Medium; BT: Biofield Treated; TI: Test item.
Assessment of Alkaline Phosphatase (ALP) Activity
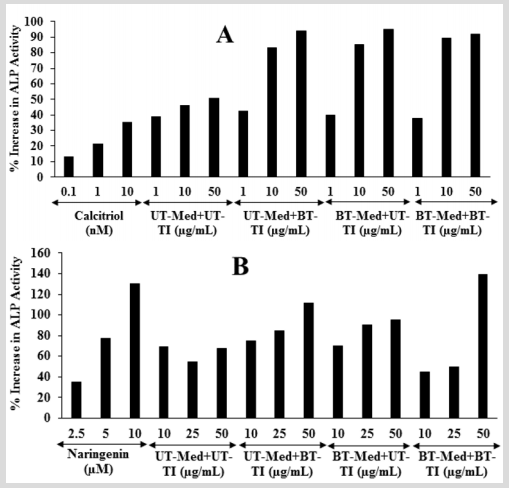
The effect of the test formulation on bone-specific alkaline phosphatase level is shown in Figure 2. The positive control, calcitriol showed 13.19%, 21.41%, and 35.37% increased the level of ALP at 0.1, 1, and 10 nM, respectively in MG-63 cells. Moreover, the experimental groups showed 80.7%, 85%, and 93.7% increased the level of ALP in the UT-Med + BT-TI, BT-Med + UT-TI, and BTMed + BT-TI groups, respectively with respect to the UT-Med + UT-TI group at 10 µg/mL. At 50 µg/mL, the percent ALP was significantly increased by 85.5%, 87.7%, and 81.5% in the UT-Med + BT-TI, BTMed + UT-TI, and BT-Med + BT-TI groups, respectively compared to the UT-Med + UT-TI group (Figure 2). Besides, the positive control naringenin showed 35.18%, 77.51%, and 130.24% increased the level of ALP at 2.5, 5, and 10 nM, respectively in Ishikawa cells. ALP percent was significantly increased by 55.5% and 65.8% in the UT-Med + BT-TI and BT-Med + UT-TI groups, respectively as compared to the UT-Med + UT-TI group at 25 µg/mL. Moreover, the experimental groups showed 66.2%, 41.8%, and 106.7% increased the level of ALP in the UT-Med + BT-TI, BT-Med + UT-TI, and BTMed + BT-TI groups, respectively with respect to the UT-Med + UT-TI group at 50 µg/mL (Figure 2). Bone-specific alkaline phosphatase (BAP) is synthesized by the osteoblast cells and is it responsible for the calcification of bone matrix. ALP is normally considered as a laboratory tool in the panel of bone and liver function tests [43]. In the case of osteoporosis the ALP activity rate is normal; while it has been increased by 2 to 4 times in case of rickets and slowly decreased by vitamin D therapy. Another literature also reported that a very high levels of ALP enzyme activity is also observed in patients with bone metastatic carcinoma and osteogenic sarcoma [44]. Here, the level of ALP was exhibited that the Biofield Energy Healing Treated novel proprietary test formulation has significantly increased the level of ALP expression, which might be very helpful to the patients suffering from various bone-related disorders.
Figure 2: The effect of the test formulation on alkaline phosphatase (ALP) in A. Human bone osteosarcoma cells (MG-63) and B. Human endometrial adenocarcinoma cells (Ishikawa). Calcitriol and naringenin were used as positive control in Mg-63 and Ishikawa cells, respectively. UT: Untreated; Med: Medium; BT: Biofield Treated; TI: Test item.
Estimation of lactate dehydrogenase (LDH) activity in human cardiac fibroblasts (HCF)
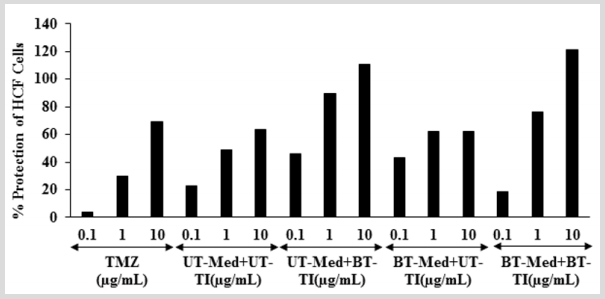
The effect of test formulation on the percent protection of HCF cells in terms of decreased level of lactate dehydrogenase (LDH) activity is shown in (Figure 3). The positive control, trimetazidine (TMZ) exhibited 3.59%, 30.14%, and 69.42% protection of HCF cells (decreased of LDH activity) compared to the t-BHP group. The percent protection of HCF cells (decreased of LDH activity) was significantly increased by 100.9% and 87.7% at 0.1 µg/mL in the UT-Med + BT-TI and BT-Med + UT-TI groups, respectively as compared to the UT-Med + UT-TI group. Moreover, at 1 µg/mL, the percent protection of HCF cells (decreased of LDH activity) was significantly increased by 83.7%, 28.4%, and 56.5% in the UT-Med + BT-TI, BT-Med + UT-TI, and BT-Med + BT-TI groups, respectively as compared to the UT-Med + UT-TI group. Further, percent protection of HCF cells (decreased of LDH activity) was also significantly increased by 74.6% and 91.2% in the UT-Med + BT-TI and BT-Med + BT-TI groups, respectively at 10 µg/mL as compared to the UTMed + UT-TI group (Figure 3). LDH is a pathologic biomarker for a wide variety of cardiovascular disorders (CVDs) such as myocardial ischemia, strenuous, etc. Various heavy metals exposure can increased the level of LDH and simultaneously more prone to CVDs [45]. The levels of LDH become high in case of cardiovascular (CBDs), hepatic (liver), and pulmonary (lungs) patients [46,47]. The study results found that there was a significant reduction of LDH level after Biofield Energy Treatment and protect heart cells, which might be helpful to resist against various pathological conditions like tissue injury, necrosis, hemolysis or malignancies, hypoxia, etc. It also indicating that the heart cells acted normally under stress and anaerobic condition and improved overall heart function.
Figure 3: The effect of the test formulation on the percent protection of HCF cells in terms of decreased lactate dehydrogenase (LDH) activity against tert-butyl hydroperoxide (t-BHP) induced damage. TMZ: Trimetazidine; UT: Untreated; Med: Medium; BT: Biofield Treated; TI: Test item.
Estimation of alanine amino transferase (ALT) activity in HepG2 cells
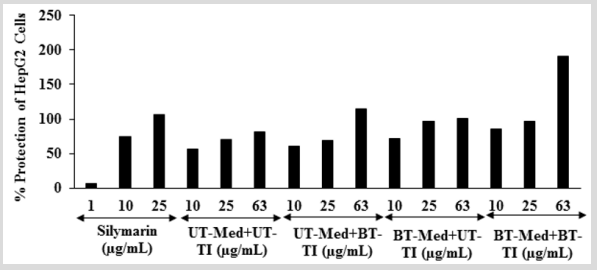
The effect of the test formulation on protection of HepG2 cells in terms of decrease alanine amino transferase (ALT) activity is shown in (Figure 4). The positive control, silymarin showed 6.56%, 74.51%, and 106.27% protection of HepG2 cells (decreased of ALT activity). The protection of HepG2 cells (decreased of ALT activity) was significantly increased by 8.2%, 27.6%, and 51.1% at 10 µg/mL in the UT-Med + BT-TI, BT-Med + UT-TI, and BT-Med + BT-TI groups, respectively as compared to the UT-Med + UT-TI group. Moreover, at 25 µg/mL, percent protection of HepG2 cells (decreased of ALT activity) was increased by 38.6% and 38.9% in the BT-Med + UT-TI and BT-Med + BT-TI groups, respectively as compared to the UTMed + UT-TI group. Further, protection of HepG2 cells (decreased of ALT activity) was also significantly increased by 39.6%, 22.8%, and 133.6% in the UT-Med + BT-TI, BT-Med + UT-TI, and BT-Med + BT-TI groups, respectively at 63 µg/mL as compared to the UTMed + UT-TI group (Figure 4). ALT is an excellent biomarker of hepatocellular injury. ALT catalyze the transfer of amino groups from alanine to ketoglutaric acid to produce oxaloacetic acid [48]. Activity of alanine aminotransferase (ALT) as an indicator of health and disease. Its activity not only measured to detect liver disease, but also to monitor overall health [49,50]. Here, the Biofield Energy Treatment significantly protect liver hepatocytes in terms of reducing the level of transaminases enzyme, ALT compared to the t-BHP inducing group, which might be due to Consciousness Energy Healing Treatment to the test formulation.
Figure 4: Effect of the test formulation on the percent protection of human liver cancer (HepG2) cells in terms of decreased alanine amino transaminase (ALT) activity under the stimulation of tert-butyl hydroperoxide (t-BHP). UT: Untreated; Med: Medium; BT: Biofield Treated; TI: Test item.
Estimation of superoxide dismutase (SOD) activity in adenocarcinomic human alveolar basal epithelial cells (A549)
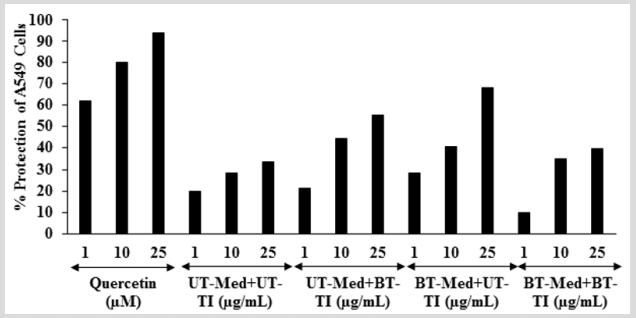
The effect of the test formulation on the protection of lungs cells (A549) in terms of increased super oxide dismutase (SOD) activity is shown in Figure 5. The positive control, showed 62.09%, 80.28%, and 93.87% protection of A549 (lungs) cells (increased of SOD activity) compared to the t-BHP group. The percent protection of A549 (lungs) cells (increased of SOD activity) was significantly increased by 8.6% and 43.8% at 1 µg/mL in the UT-Med + BT-TI and BT-Med + UT-TI groups, respectively compared to the UT-Med + UT-TI group. Moreover, at 10 µg/mL, the percent protection of A549 (lungs) cells (increased of SOD activity) was significantly increased by 55.8%, 42.2%, and 22.3% in the UT-Med + BT-TI, BT-Med + UT-TI, and BT-Med + BT-TI groups, respectively as compared to the UTMed + UT-TI group (Figure 5). Oxidative stress is a crucial causative factor for lung disorder like chronic obstructive pulmonary disease (COPD). Superoxide dismutases (SODs) can prevent an increase in oxidative burden by converting the superoxide radicals to hydrogen peroxide [51,52]. Altogether, data observed that a significant increased SOD level after Biofield Energy Treatment in A549 cells, which might be helpful to resist against various pathological conditions like oxidative stress and related adverse effect. It also indicating that the lung cells acted normally and improved overall respiratory activities.
Figure 5: Effect of the test formulation on the percent protection of lungs cells (A549) in terms of increased SOD activity under the stimulation of tert-butyl hydroperoxide (t-BHP). UT: Untreated; Med: Medium; BT: Biofield Treated; TI: Test item.
Effect of test formulation on serotonin in human neuroblastoma (SH-SY5Y) cells
Figure 6: Effect of the test formulation on percent increase in 5-hydroxy tryptamine (5-HT) or serotonin in human neuroblastoma cells (SH-SY5Y). UT: Untreated; Med: Medium; BT: Biofield Treated; TI: Test item.

The effect of test formulation on the level of serotonin in SHSY5Y cells is shown in Figure 6. The positive control, showed 98.2%, 123.53%, and 156.76% increased the level of serotonin. The level of serotonin was significantly increased by 58% and 80% in the BT-Med + UT-TI and BT-Med + BT-TI groups, respectively at 10 µg/mL compared to the UT-Med + UT-TI group. Moreover, at 25 µg/mL, 5-HT level was significantly increased by 17.6%, 31.7%, and 77.2% in the UT-Med + BT-TI, BT-Med + UT-TI, and BT-Med + BT-TI groups, respectively as compared to the UT-Med + UT-TI group. Further, the serotonin level was significantly increased by 43.3%, 53.2%, and 58.7% in the UT-Med + BT-TI, BT-Med + UT-TI, and BT-Med + BT-TI groups, respectively at 63 µg/mL as compared to the UT-Med + UT-TI group (Figure 6). Serotonin (5- HT) is a neurotransmitter responsible for stress, anxiety, aggressive behavior, and for the regulation of blood pressure Loss of 5-HT leads to various neuropsychiatric disorders such as depression, memory loss, Alzheimer’s disease, cognitive health, loss of ability of thinking, etc. Several studies have been reported that mood change directly link with the level of serotonin. For example, lower mood is due to lower platelet serotonin2 receptor function or lower level of serotonin; whereas better mood is associated with higher blood serotonin levels [53,54]. Apart from brain tissue serotonin can impact on hematopoietic system, immune responses, and tissue regeneration support utilization of serotonin as a potential therapeutic target for the treatment of hematological diseases and organ repair [55]. Here, in this study, the Biofield Energy Treated novel test formulation and Biofield Energy Treatment per se have significantly increased the level of serotonin, which might beneficial for various neurodegenerative diseases and other age-related disorders and improved the normal functioning of the brain tissues i.e., brain health.
Effect of test formulation on vitamin D receptors (VDRs)
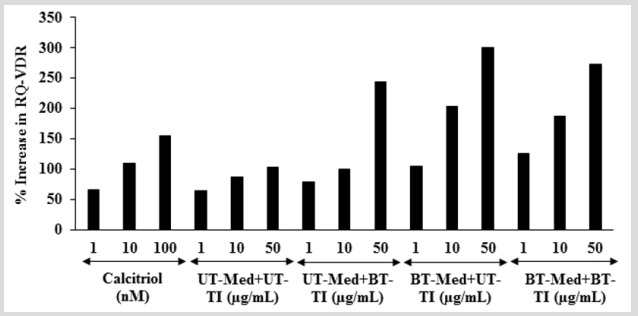
The effect of the test formulation on VDR expression was determined using quantitative-polymerase chain reaction (Q-PCR) amplification in human bone osteosarcoma cells (MG-63). VDRrelative threshold cycle (VDR-CT) values were obtained from PCR amplification. Relative quantification (RQ) was calculated from the VDR-CT and house-keeping (HK)-CT values for MG-63 cells treated with test formulation and positive control is shown in Figure 7. The positive control (calcitriol) showed 65.86%, 109.94%, and 154.91% increase of RQ of VDR at 1, 10, and 100 nM, respectively. Moreover, RQ of VDR was significantly increased by 22.1%, 61%, and 95.6% in the UT-Med + BT-TI, BT-Med + UT-TI, and BT-Med + BT-TI groups, respectively at 1 µg/mL compared to the UT-Med + UT-TI group. Additionally, at 10 µg/mL the VDR level was significantly increased by 15.5%, 134.6%, and 115.7% in the UT-Med + BT-TI, BT-Med + UT-TI, and BT-Med + BT-TI groups, respectively as compared to the UT-Med + UT-TI group. Further, VDR level was also significantly increased by 136.8%, 191.9%, and 165.8% in the UT-Med + BT-TI, BT-Med + UT-TI, and BT-Med + BT-TI groups, respectively at 50 µg/ mL compared to the UT-Med + UT-TI group. VDRs are found in all types of the skeleton such as osteocytes, chondrocytes, osteoclasts, and osteoblasts. Deficiency of vitamin D is due to lack of functional VDRs, which can be reverse using diets containing more calcium and phosphate [56,57]. Here, in this experiment the Biofield Energy treated test formulation has significantly increased the expression of VDRs, which might be helpful to maintain adequate vitamin D level for the normal growth and development of bone cells.
Figure 7: Effect of the test formulation on percent increase in relative quantification (RQ) of vitamin D receptors (VDRs) gene in human bone osteosarcoma cells (MG-63). UT: Untreated; Med: Medium; BT: Biofield Treated; TI: Test item.
Conclusion
The study findings showed that the tested novel test formulation was safe and non-toxic based on MTT cell viability assay in six tested cells. The BT-Med + BT-TI group showed 53.5%, 127.9%, and 53.3% restoration of cell viability at 0.1, 10, and 25 µg/mL, respectively in human cardiac fibroblasts cells (HCF) compared to the UT-Med + UT-TI group. Moreover, the BT-Med + BT-TI group showed 72.6% and 67.7% restoration of cell viability at 0.1 µg/mL in human hepatoma cells (HepG2) and adenocarcinomic human alveolar basal epithelial cells (A549), respectively compared to the untreated group. Alkaline phosphatase (ALP) activity was significantly increased by 80.7%, 85%, and 93.7% in the UT-Med + BT-TI, BT-Med + UT-TI, and BT-Med + BT-TI group at 10 µg/mL in human bone osteosarcoma cells (MG-63). Moreover, ALP activity was significantly increased by 106.7% in the BT-Med + BT-TI group at 50 µg/mL than untreated group. The percent protection of HCF cells (decreased of LDH activity) was significantly increased by 100.9% (at 0.1 µg/mL) and 91.2% (at 10 µg/mL) in the UT-Med + BT-TI and BT-Med + BT-TI groups, respectively compared to the untreated group in HCF cells. The percent protection of HepG2 cells (decreased of ALT activity) was significantly increased by 133.6% at 63 µg/mL in the BT-Med + BT-TI group compared to the untreated group in HepG2 cells. The percent protection of A549 (lungs) cells (increased of SOD activity) was significantly increased by 55.8% in the UT-Med + BT-TI group at 10 µg/mL compared to the untreated group in A549 cells. The serotonin level was significantly increased by 80%, 77.2%, and 58.7% in the BT-Med + BT-TI group at 10, 25, and 63 µg/mL, respectively compared to the untreated group in human neuroblastoma cells (SH-SY5Y). The relative quantification (RQ) of vitamin D receptors (VDRs) level was significantly increased by 136.8%, 191.9%, and 165.8% in the UT-Med + BT-TI, BT-Med + UT-TI, and BT-Med + BT-TI groups, respectively compared to the untreated group in MG-63 cells. In conclusion, The Biofield Energy Treatment significantly improved heart, liver, bones, neuronal, and lungs related functional enzyme biomarkers and also protected cardiomyocyte, hepatocyte, osteocytes, pneumocyte, and nerve cells from oxidative damage induced by tert-butyl hydroperoxide (t-BHP). Thus, results suggested that Biofield Energy Treatment can be used as a complementary and alternative treatment for the prevention of various types of cardiac disorders (peripheral artery disease, high blood pressure, congenital heart disease, stroke, congestive heart failure, rheumatic heart disease, carditis, valvular heart disease, thromboembolic disease, and venous thrombosis, etc.), hepatic disorders (cirrhosis, Wilson disease, liver cancer, hemochromatosis), and lungs disorders (Asthma, Emphysema, Chronic bronchitis, Pneumonia, Cystic fibrosis). Further, it can be useful to improve cell-to-cell messaging, normal cell growth and differentiation, cell cycling and proliferation, neurotransmission, skin health, hormonal balance, immune and cardiovascular functions. Moreover, it can also be utilized in organ transplants (i.e., liver, kidney, and heart transplants), aging, hormonal imbalance and various inflammatory and immune-related disease conditions like Alzheimer’s Disease (AD), Dermatitis, Asthma, Ulcerative Colitis (UC), Hashimoto Thyroiditis, Pernicious Anemia, Sjogren Syndrome, Aplastic Anemia, Multiple Sclerosis, Hepatitis, Graves’ Disease, Irritable Bowel Syndrome (IBS), Dermatomyositis, Diabetes, Myasthenia Gravis, Atherosclerosis, Parkinson’s Disease, Systemic etc. to Lupus Erythematosus (SLE), stress, improve overall health and Quality of Life.
Acknowledgement
Authors gratefully acknowledged to Trivedi Global, Inc., Trivedi Science, and Trivedi Master Wellness for their support. In addition, authors are thankful for the support of Dabur Research Foundation for conducting this study.
For
more Lupine Publishers Open Access
Journals Please visit our website:
http://lupinepublishers.us/
For more Research
and Reviews on Healthcare articles Please Click Here:
https://lupinepublishers.com/research-and-reviews-journal/
Lupine Publishers: Lupine Publishers | TRACK Implementation Among Ban...
Thursday, October 7, 2021
Lupine Publishers: Lupine Publishers| Prevalence of Obesity and Assoc...
Wednesday, October 6, 2021
Lupine Publishers: Lupine Publishers| A Simple Mathematical Model for...
Friday, October 1, 2021
Lupine Publishers: Lupine Publishers| A Simple Mathematical Model for...
Lupine Publishers|Spontanous Human Combustion-Are the Microzymas The Culprit?
Lupine Publishers | Journal of Health Research and Reviews
Short Communication
Spontaneous human combustion is a contentious and extremely rare phenomenon whereby a person suddenly bursts into flames or catches on fire without any visible external stimuli such as petrol and matches etc. being applied to the person. The victim often burns to ashes, yet the surroundings remain unburnt/unscorched/ unscathed and the fire is promptly extinguished as miraculously as it started! Could there be a rational, scientific explanation for these freaky fires, scores of which have been documented in the last half millennium? Skeptics of this phenomenon abound, and some cases attributed to spontaneous human combustion have been argued to be due to concealed cigarette butts etc. But not all cases of this phenomenon can be so readily dismissed [1-4].
Picture a spherical living entity, too small to be seen by the naked eye. A living entity smaller than bacteria, viruses, red blood cells, white blood cells, fungi and protozoa. These living motile entities cannot be destroyed by any means yet devised by man. They are found in all living things-human beings, plants, dogs, lions, ostriches, giraffes etc. Once it’s alive-the entity is found therein. These entities are found in the air also. These entities sometimes come together in groups and clumps and form bacteria. In greater agglutinations they form cells and tissues. And surprisingly when an animal or human being ceases to exist, these tiny bodies continue to live! Welcome to the world of the microzymas also known as cellular dust [5-7] theorized to be the creators of life on earth, and of the entire universe [8].
Under normal circumstances, heat in the human body is produced by basic metabolic processes, specific dynamic action and muscular activity. Heat is lost from the body by radiation, conduction, sweat vaporization, respiration and urination. It could very well be that in rare instances microzyma carry out some extremely exothermic reactions for which tissue conductance is hopelessly inadequate, and thus the cells, tissues, organs and systems literally burn up! The death of the unfortunate individual coincides with the de-coordination of the microzyma and that explains why the fire doesn’t affect inanimate objects around the victim to an appreciable degree. Barring ketosis, the wick effect, ball lightning and supernatural forces, microzyman reactions gone awry i.e. “rogue” microzymas might prove to be a rational explanation for the very perplexing spectacle called spontaneous human combustion.
For
more Lupine Publishers Open Access
Journals Please visit our website:
http://lupinepublishers.us/
For more Research
and Reviews on Healthcare articles Please Click Here:
https://lupinepublishers.com/research-and-reviews-journal/
Lupine Publishers: Lupine Publishers| A Standard Pediatric Dental Clinic
Lupine Publishers: Lupine Publishers| A Standard Pediatric Dental Clinic : Lupine Publishers| Journal of Dentistry and Oral Health Care Aft...
-
Lupine Publishers: Lupine Publishers| A Standard Pediatric Dental Clinic : Lupine Publishers| Journal of Dentistry and Oral Health Care Aft...
-
Lupine Publishers | Journal of Health Research and Reviews Abstract Metabolism is the process your body uses to make energy f...
-
Lupine Publishers | Journal of Health Research and Reviews bstract Purity of the person is guarantee of his health. Spiritual and...