Lupine Publishers | Journal of Health Research and Reviews
Abstract
Neurodegeneration with brain iron accumulation (NBIA) is a rare but well-established disorder of the central nervous system characterized by abnormal accumulation of iron in the basal ganglia. Upon presentation, the underlying specific defect can be deduced from the signs/symptoms and clinical presentation of the patient and thus can be placed in a specific category. However, idiopathic cases do exist, albeit very rare. In this case report, we present a patient with idiopathic NBIA, her clinical and genetic results did not match any of the six known types of NBIA. We also examine ways of differentiating NBIA types, and the crucial role of genetic and radiological tests in their diagnosis. Although, genetic testing can provide a definite diagnosis, but a combination of radiological and clinical features are quite helpful in supporting the diagnosis of NBIA.
Keywords: Neurodegeneration; Eye-of-The-Tiger sign; Basal ganglia; Aceruloplasminaemia; Neuroferritinopathy
Introduction
Neurodegeneration with brain iron accumulation (NBIA) is a collection of neurodegenerative motor disorders characterized by extrapyramidal features with a) one or more of dystonia, rigidity and/or choreoathetosis, and b) accumulation of iron in the brain, especially in the basal ganglia (BG). It is composed of seven members of movement disorders: classical pantothenate kinaseassociated neurodegeneration (C-PKAN), atypical PKAN (A-PKAN), infantile neuroaxonaldystrophy (I-NAD), atypical NAD (A-NAD), aceruloplasminaemia, neuroferritinopathy, and idiopathic NBIA [1]. The estimated prevalence of NBIA is roughly 1-3/1,000,000. Magnetic resonance imaging (MRI) and genetic testing are considered the gold standard when performing a differential diagnosis for NBIA. Unfortunately, genetic tests for candidate mutated genes are not always accessible to the clinician, thus greatly impeding diagnosis of the disease. In lieu of genetic tests, radiological and clinical features become important. Therefore, this case report delves into the diagnostic potential of radiological and clinical features of NBIA during differential diagnosis of its various forms, and stresses that genetic tests for NBIA be accessible to all neurologists.
Case Report
A 31-year-old right-handed female who began noticing trouble with her speech initially eight years ago, which became slow, stiff, and unable to maintain tone of her speech. She also developed drooling that occurred intermittently. Initially, she had no history of cognitive dysfunction but developed intellectual decline gradually over the next two years. She then noticed slowing down in her activities of daily living. She also developed unsteadiness on her feet. She had intermittent tremor of her left hand while holding things and during action. She developed facial dystonia with a fixed smile over the last few years. Her family noticed a gradual decrease in her social life but she is independent in activities of daily living. Gradually, she required some help with her basic activities of daily life. She was not able to manage her finances and required her sister’s assistance in this regard. On examination, she had spastic dysarthria and a fixed smile on her face characteristic of facial dystonia. Motor testing showed rigidity of upper and lower extremities, and dystonic posture of fingers of both hands. She had moderate bradykinesia on both sides and difficulty performing repetitive movements. There was no resting tremor but she had large amplitude flapping movements of both upper extremities in wing beating position. There was a wide amplitude kinetic tremor of both upper extremities without target accentuation. Deep tendon reflexes were diminished throughout, except they were 1+ in left biceps and brachioradialis. Plantar stimulations were extensor on both sides. Examination of gait showed that both arms and fingers were held in dystonic position. She had dystonic inversion and slight plantar flexion especially of the left leg. Her base was wide. She was turning slowly.
Radiological Investigation
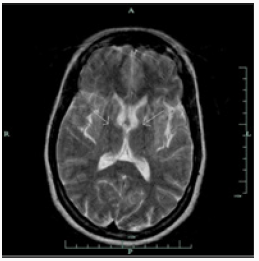
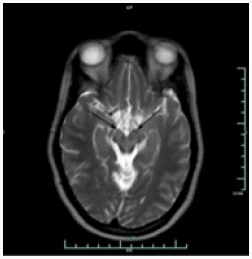
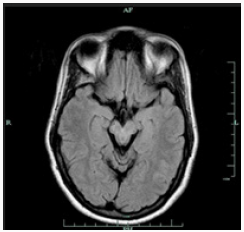
Brain MRI shows bilaterally symmetrical hypointensity in the globus pallidus (Figure 1), hypointensity in the substantia nigra (Figure 2), especially in the caudate head and putamen, and hyperintensity in midbrain cerebral peduncles (Figure 3). There were areas of abnormal signal intensity identified in the periventricular white matter and the anterior aspect of the corona radiata in right frontal lobe. This demonstrated low signal intensity on T1-weighted images and increased signal intensity on FLAIR and T2-weighted images. There was no abnormal enhancement with gadolinium and no hemosiderin deposition was seen. There were also smaller foci of abnormal signal intensity located in the right thalamus, periventricular white matter in the left hemisphere, anterior limb of the left internal capsule, posterior aspect of the pons, and midbrain on the right side. The patient initially presented with symptoms at the age of 23 and was eventually diagnosed with idiopathic NBIA at age of 28. She had no known family history of NBIA.
Discussion
Gregory et al. [1] divide NBIA into those with a) early onset and rapid progression and b) late onset and slow progression. This categorization includes classical (a) and atypical PKAN (b), infantile and atypical NAD (a), neuroferritinopathy (b), aceruloplasminaemia (b), and idiopathic NBIA (a, b). PANK2 mutations localized on chromosome 20p13-12.3 lead to PKAN. Since half of patients diagnosed with NBIA present mutations in PANK2, this makes PKAN the most common form of NBIA [1]. Homozygous mutations undertake base substitution from adenine-764 to guanine [2], whereas heterozygous mutation substitute cysteine-1184 and guanine-1238 with adenine [3]. Moreover, mutations take place through various routes including deletion, allele loss-offunction, and missense mutations [4]. Neuroferritinopathy and aceruloplasminaemia are results of ferritin light polypeptide [5] and ceruloplasmin mutations [6], respectively. Infantile NAD and atypical NAD are caused by mutations in PLA2G6 (calciumindependent group VI phospholipase A2) [1]. Genetic test for PANK2 was negative for our patient, hence ruling out PKAN. Due to inaccessibility of genetic tests for I- and A-NAD, neuroferritinopathy, and aceruloplasminaemia, clinical and radiological symptoms became critical when performing a differential diagnosis. Our patient presented initial symptoms at approximately 23 years of age and was confirmed for NBIA at the age of 28. Both I- and A-NAD can safely be ruled out since their onset is much earlier (i.e., infantile) at 2 and 4.4 years of age, respectively. Both conditions are also accompanied by psychomotor decline, optic degeneration, early truncal hypotonia, tetraparesis, and generalized seizures [1], none of which were presented by our patient. Ferritinopathy is associated with choreoathetosis, spasticity, dystonia, and rigidity [7]. Except for facial dystonia and dystonic posture of her fingers, none of the aforementioned signs were observed during examination. Upon further investigation, she had normal serum ferritin level [42UG/L] which is significantly decreased in cases of ferritinopathy. In addition to normal ferritin levels, serum ceruloplasmin [430MG/L], serum copper [13UMOL/L] urine copper [0.13UMOL/D], AST [17], ALT [12] and iron [15UMOL/L] levels were also normal. Iron binding capacity was 61UMOL/L with iron saturation of 0.25. Our patient did not exhibit retinal degeneration nor diabetes mellitus which are commonly seen in aceruloplasminaemia, along with elevated ferritin level, reduced copper and iron concentrations in the serum [1,8]. Upon ruling out the aforementioned forms of NBIA and not finding evidence to suggest otherwise, a diagnosis of idiopathic NBIA was entertained. Nevertheless, because the “eyeof- the-tiger” sign on T2 weighted brain MRI is so common among various types of NBIA, as observed in our patient (Figure 3), it is worthwhile noting other radiological features of different forms of NBIA.
Patients who are positive for PANK2 mutation always exhibit the “eye-of-the-tiger” sign on T2-weighted brain MRI. Clinical diagnosis of NBIA requires hypointensity in GP, SN, and dentate nuclei (DN). Central hyperintensity is observed in the globus pallidus (GP), surrounded by hypointensity due to iron accumulation [9]. Iron accumulation is also observed in the reticular region of the substantianigra (SN). However, hypointensity from iron accumulation can be mistaken for calcium when viewed from T2- weighted MRI. Such distinction can be clarified by taking a T1- weighted MRI wherein isointense signal from GP and SN would substitute hypointense signals observed in T2-weighted images or a CT scan which would show calcification being hyperdense. While patients with mutation in PANK2 show the “eye-of-the-tiger” sign, the opposite may not be always the case; our patient presented the latter scenario, as other reports have also described [4]. GP is still a key structure when diagnosing NBIA even when hypointensity is not observed. Instead, isolated areas of hyperintensity are visible in GP that can lead to early diagnosis. Interestingly, DN hypointensity is only observed in patients and can be viewed by T2-weighted and fast spin echo MRI [9]. PKAN covers only half of cases in NBIA, therefore caution is required when performing differential diagnosis among NBIA other than PKAN just by radiological investigation. NAD involves hypointensity in GP and SN when viewed under T2- weighted and FSE MRI, and in DN when viewed under T2-weighted MRI only. On the other hand, the putamen in addition to DN and GP are involved in neuroferritinopathy with almost half of patients showing hyperintensities areas in the pallidum and putamen. Such areas are not observed in aceruloplasminemia. Rather, diffuse damage takes over BG and thalami [9]. It is an extremely rare for a patient to have neurodegeneration due to brain iron accumulation, but what is even rarer is for a patient to have the idiopathic form of it. NBIA is usually associated with a genetic history and is seen in family members. Also, this case report describes a patient with no family history of the disease. It is usually a patient with the PANK2 mutation that has the ‘eye-of-the-tiger’ sign on MRI, but the patient in the case report had the sign while having the idiopathic form of the disease [10]. This case report emphasizes the importance of genetic testing and clinical signs/symptoms in diagnosing NBIA.
Acknowledgment
Authors acknowledge help of Medhat Chowdhury and Atif Saeed Khan in formatting this paper.
For more Lupine Publishers Open
Access Journals Please visit our website:
http://lupinepublishers.us/
For more Research
and Reviews on Healthcare articles Please Click Here:
https://lupinepublishers.com/research-and-reviews-journal/
To Know More About Open Access
Publishers Please Click on Lupine
Publishers
Follow on Linkedin : https://www.linkedin.com/company/lupinepublishers
Follow on Twitter : https://twitter.com/lupine_online



No comments:
Post a Comment