Lupine Publishers | Journal of Health Research and Reviews
Abstract
Keywords: Ketoconazole; Self emulsifying drug delivery system (SEDDS); Medicated stick; Fungal diseases; Beautification; Excipients; Optimization; Evaluation; Stability
Cosmetics
Cosmeceutical
Introduction
Ketoconazole have low solubility and high permeability. It is readily but incompletely absorbed after oral dosing and is highly variable due to its poor water solubility leading to shorter half-life i.e. 2 h of the drug. Topically it is used in the treatment of candidal or tinea infections of the skin. Self Emulsifying Drug Delivery System (SEDDS) have been used as drug carrier in topical treatment of diseases, especially in dermatology. This system allows for a high accumulation of drug in the skin, with relatively low permeation flux as compared to the conventional dosage form. SEDDS enhance surface area and so it improve solubility of Ketoconazole, thus drug was easily soluble in formulation then use SEDDS in stick formulation it was enhance bioavailability.
Main objective is to prepare solid self emulsifying drug delivery system containing Ketoconazole using appropriate oil, surfactants and co-surfactants to improve solubility. The formulation of Ketoconazole drug contain medicated Stick is intended for the purpose of beautification of lips and curing lip with fungal infections. For this rational it was formulate one cannot go outside applying Ketoconazole cream on lip but applying Ketoconazole medistick is better. Medistick contain lake oil soluble colour pigment they does not interfere in formulation and also use as medistick and transparent medistick use for other body organ which are infected from fungal like eyebrows, neck, ear back side this type site it is apply. Ketoconazole is very safer drug it is not harmful even if people or child eats. Medistick is cosmetic formulation for the modification of colour and prepared by moulding a dispersion of colours in a waxy base, in the form of stick [4].
Topical Drug Delivery System
Figure 1.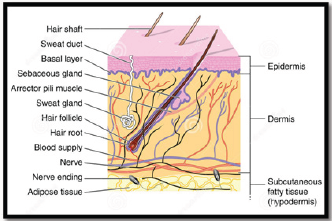
- i. External delivery that are rolling stick ,spread, sprayed or
otherwise isolated on to cutaneous tissues to cover the infected area.
ii. Internal delivery that are applied to the mucous membrane orally, virginally or rectal tissues for local activity.
- a. Merits
i. It is convenient to apply and use and avoids first pass metabolism.
ii. The risk of inconvenient intravenous therapy is avoided and also varied condition of absorption it avoided, like pH changes, presence of enzymes, gastric emptying time etc.
iii. It contains ability to deliver drug more selectively to specific site and avoids gastro-intestinal incompatibility.
iv. Utilization of drugs with short biological half-life, narrow therapeutical window and Improves physiological and pharmacological response and patient compliances.
v. It is suitable for self-medication and is easy to terminate the medications, when needed.
vi. Efficiency is achieved throw lower total daily dosages of drug by inputting continuous drug.
vii. It avoids changes in drug levels, inter &intra patient variations.
viii. It is applied on large area as compare to buccal or nasal cavity.
- b. Demerits
i. Skin irritation may occur due to the drug and/or excipients on contact dermatitis.
ii. Due to poor permeability of some drugs possibility of allergenic reactions may caused through the skin.
iii. Enzyme present in epidermis may change the nature drugs.
iv. Larger size of drugs particles is not easy to absorb by the skin.
Material and Method
Material
a. For Seddsi. Oils
Long chain triglyceride and medium-chain triglycerides oils with proportionate degree of saturating have been used in the design of SEDDS. Recent novel semi synthetic medium chain triglyceride oils have sufficient property and vastly replace regular medium chain triglyceride. Oleic acid which is use in formulation.
ii.Surfactants
Invariably a surfactant or emulsifier having a good binding force to attach SEDDS within. High number of hydrophobic drugs can be dissolve in naturally obtained surfactant due to its amphiphilic property. But emulsification process needs two big issues to be satisfied, HLB and Safety. If one needs higher emulsification then HLB value should be greater (high hydrophilicity). High emulsification of SEDDS Formulations prevents drug precipitation in gastro intestinal lumen ( due to raped o/w droplet formation ) side by side prolong duration of action would be assure. Non-ionic surfactants are also considered as safer than the ionic ones. The surfactant concentration should be in the range of 30-60%w/w while dissolving high amount of hydrophobic drugs.
But high concentration of surfactant can irritate GI lumen that could be a big challenge for pharmaceutical scientist. However augmentation of particular droplets at critical concentration is amenable during high concentrated emulsification process, due to this the interfacial disruption caused by enhanced water penetration into the oil droplets, mediated by the increased surfactant concentration, thus leading ultimately to the ejection of oil droplets into the aqueous phase.
iii.Co surfactants/co-solvents
The production of an optimum SEDDS requires relatively high concentrations (generally more than 30% w/w) of surfactants. Organic solvents such as, ethanol, propylene glycol (PG), and polyethylene glycol (PEG) are suitable for topical drug delivery, and they enable the dissolution of large quantities of either the hydrophilic surfactant or the drug in the lipid base. These solvents can even act as co-surfactants in SEDDS systems. On the other hand, alcohols and other volatile co-solvents have the disadvantage of evaporating into the shells ofthe soft or hard gelatine, sealed gelatine capsules in conventional SEDDS leading to drug precipitation. Thus, alcohol free formulations have been designed, but their lipophilic drug dissolution ability may be limited. Rapid emulsification occurs on optimised 35%w/w concentration. Systems containing 20-50% w/w Tween 85 are very fatly in nature. 50%w/w accelerates, produce viscous gels. By homogenisation technique fine dispersion could be possible. Advance is Transcutol P which one used.
b. For Medistick
i.Waxes
Waxes The gloss & hardness are generally depends on characteristics & quantity of waxes Best characteristic is obtained by using mixture of waxes of different m.p & adjusting the final melting point by incorporating a sufficient amount of high melting point wax: beeswax, carnauba wax, hard paraffin wax, soft paraffine, lanolin etc
ii.Oils
The oil mixture is required to blend properly with the waxes to provide a suitable film on the applied lip skin. Also acts as solvent in some formulation, acts as dispersing agent for insoluble pigments the ideal mixture of oil should produce the product, easily spread & produce a thin film with good covering power. Examples: Castor oil, Tetrahydrofurfuryl alcohol (THFA) & esters, Isopropyl myristate, Isopropyl palmitate, Butyl stearate.
iii.Colors
Colors most important from commercial & appearance point of view. In olden days, carmine was widely used, but nowadays various other are available. Color in lipstick is imparted by two ways: Soluble dyes: By staining the skin with soln of dyestuff which can penetrate the outer layer of skin. Insoluble dyes by covering the lips with a colored layer which serves to hide any skin roughness & give a smooth appearance.
iv. Preservatives
Preservatives used to prevent microbial growth Example: 0.1% Propyl parahydrohybenzoate in 0.1% higher conc. of preservative can cause slightly burning sensation or allergic reaction.
v.Fragrance
Fragrance Essential component of medistick used to mask bad odour of fatty or wax. Used to impart attractive flavour Conc. 2-4% Qualities for selection: Free from irritating effect Free from disagreeable taste Stable.
vi. Antioxidants
Antioxidants Incorporated to prevent Rancidification of oily base during storage. Generally used in combination Example: BHA, BHT, Propyl gallate, Citric acid, Surfactants & Other Additives
Surfactants: Used to promote wetting & stabilize the dispersion of insoluble pigments in medistick base.
Additives: used for various purposes
Oil - soluble sunscreen: filter the sunrays & protect lip skin from sun burn.
Silicon fluid: used as fixative & prevent colors, from bleeding on lips.
PVP: (conc. 0.5-1%) film former on lips & reduce allergic reaction in medistick.
Isopropyl linoleate: prevent drying effect.
Method
Preparation of liquid SEDDS formulation
For making 2% Ketoconazole Medistick requires 200mg API. The plans were set up by dissolving the detailing measure of 2% Ketoconazole (0.2g) in the blend of oil (Oleic Acid), surfactant (Tween 80), co-surfactant (Transcutol-P) at 37 °C. The blend was homogenized with mixing with the help of magnetic stirrer until drug completely soluble. It is liquid SEDDS. Optimize the SEDDS.Preparation of Medistick: (Table 1)[5]
Table 1.
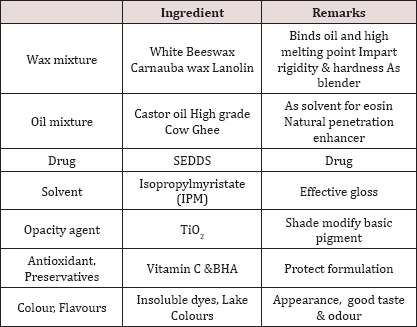
- i. Weight wax mixture, oil mixture, solvent, antioxidant, preservatives, TiO2, flavour & colours all the ingredients.
ii. Take wax mixture in a china dish and put on the water bath till wax mixture dissolve and Take a beaker into other water bath for warming oil mixture.
iii. Add wax mixture and SEDDS into oil mixture with continues stirring.
iv. Take the solvent of IPM, colours, flavours, antioxidant &preservatives and TiO2in another beaker and mix properly.
v. Pour the 4) into 3) with continuously stirring and then mould it in medistick moulders. First Cool it at room temperature then freeze & scrap it out.
Evalution Parameters [6-9]:
- a. Color: Visual technique
b. pHparameter: The pH of defined natural was resolved utilizing computerized pH meter.
c. Skin disturbance test: It is followed by applying medistick on the skin for 10 min.
d. Solubility test: To check the solubility of medistick in different solvents.
e. Perfume test: The medistick were tested after 30 days, to record fragrance.
f. Melting Point: Medistick sample was filled into a glass capillary tube open on both the closures. This tube was cooled in ice and secures it to a thermometer. This assembly was immersed into a beaker full of water and was warmed with nonstop mixing. The temperature at which the material moves along capillary tube was viewed as its dissolving point.
g. Breaking Load test: Breaking point was done to determine the strength of medistick. The medistick was held horizontally in a socket at the midpoint from the edge of help. The weight was continuously expanded by a specific value (10 gm) at specific interval of 30 second and weight at which medistick breaks was considered as the breaking point.
h. Softening Point: The medistick sample inserted in to an aluminium ring, after removing extra mass above and below the orifice. This was placed in a fridge (6°C) for 10 min. After removing it from the fridge, the ring was fastened onto a stand. This assembly was dipped in to a beaker full of water. This was heated with a constant stirring. Temperature was observed utilizing a thermometer. Softening point was the temperature at which the medistick mass was starting to melt and falls into the beaker.
i. Stability Studies: The medistick were placed for stability studies at room temperature, Refrigerator and 40 ± 2 °C/75 ± 5% RH and were observed for any physical changes.
j. Permeability Study: Cellophane membrane was soaked in ethanol and allowed to evaporate. 50 mg of medistick mass was applied on the membrane and it was placed on the diffusion cell. Hydro alcoholic Mixture (30% ethanol in distilled water %v/v) was used as receptor media. This was magnetically stirred (600rpm).The experimental temperature was kept up at 32 °C by circulation thermostatic water inside the cell jacket. Sampling was done at 1hr interval and analyzed under UV at 283nm for 6hrs.
k. Thixotrophy character: It is sign of thixotropic quality and is finished by utilizing penetrometer. A standard needle of specific diameter is allowed to penetrate for 5 seconds under a 50 gm load at 25 °C. The depth of penetration is a measurement of the thixotropic structure of to be 10.5mm.
l. Antifungal activity: Ketoconazole is fungistatic and inhibits the biosynthesis of ergosterol, the major sterol found in the fungal cell layer. The antifungal activity of ketoconazole from the formulation as well as from standard (Drug dissolved in 30% v/v of ethanol) was determined using Candida albicans as a representative fungus; by the cup plate method. It determines antifungal activity of inhibition zone.
m. Force of application: It is test for comparative measurement of the force to be applied for application. A piece of coarse brown paper can be kept on a shadow graph balance and medistick can be applied at 45o angle to cover a 1 sq. inch area until fully covered. The pressure reading is an indication of force of application and it depends on the operator.
n. Surface anomalies: It is study of the surface defects, such as formation of crystals on surface, contamination by fungus etc. No surface anomalies must be recorded in the formulated medistick.
o. Rancidity: It rancidification is the decomposition of fats, oils and other lipids by hydrolysis or oxidation. Is the oxidation of castor oil or other waxy or lipoidal ingredients? It leads to obnoxious odour, bad taste & sticky product & sometimes changes of colour of the product. Testing of rancidity can be done by determining its peroxide number.
p. Rupture Test: Rupture test medistick is placed in two holders, in the extended position. Weight is added to the holder on the medistick portion at 30-second intervals until the medistick ruptures. The pressure required to rupture the medistick is then checked against the manufacturer's standards. Since there are no industry standards for these tests, each manufacturer sets its own parameters.
q. Spreadability Test: Medistick was spread over transparent glass in angle of 45°. The surface was observed and the picture was taken with dark background. Good spreadability was seen.
r. Calibration curve: Accurately weight 10mg of Ketoconazole was transferred to a 100ml volumetric flask and dissolved in 60ml methanol. The volume was adjusted to the mark with methanol to prepare stock solution (100mcg/ ml) the above solution is further diluted with methanol to get concentration of Ketoconazole in the range of 3-15mcg/ml.Absorbance of each solution was measured at 222nm against a reagent blank solution prepared similarly without drug UV spectrophotometer. Then plot the concentration versus absorbance graph. Same do for phosphate butter pH 7.4.
Conclusion
For more Lupine Publishers Open Access Journals Please visit our website:
http://lupinepublishers.us/
For more Research and Reviews on Healthcare articles Please Click Here:
https://lupinepublishers.com/research-and-reviews-journal/
To Know More About Open Access Publishers Please Click on Lupine Publishers
Follow on Linkedin : https://www.linkedin.com/company/lupinepublishers
Follow on Twitter : https://twitter.com/lupine_online



No comments:
Post a Comment