Lupine Publishers | Journal of Health Research and Reviews
Abstract
Keywords: Crystallo co agglomeration; Good solvent; Bridging liquid; Micromeritics
Abbreviations: CCA: Crystallo Co Agglomeration; GIT: Gastro-Intestinal Tract; CS: Crushing Strength; Tc: Crystallization Temperature; PVP: Polyvinyl Pyrrolidone; HPMC: Hydroxy Propyl Methylcellulose; PEG: Poly Ethylene Glycol; EC: Ethyl Cellulose; PVP: Poly Vinyl pyruvate
Introduction
The properties of powder material associated with the plan assume a basic part in compelling granulation and after that onwards in pressure into tablets. The tablets might be defined by wet granulation, pressure granulation and direct pressure. The procedure, for example, wet granulation and pressure granulation includes more advances and time to wrap up. The materials which are not steady under warmth and dampness may not be reasonable to process through more prevalent system of wet granulation. Until the late 1950's, the majority of tablets were produced in the world by means of granulation techniques and subsequent compression [1,2]. The essential target of granulation is to deliver free streaming and exceptionally compressible blend of fixings. The excipients should indicate great stream, union and greasing up properties under strain to make such compacts.
The accessibility of new types of excipients and altered hardware and additionally advances enabled the conceivable outcomes to create tablets by coordinate pressure. The direct compression was long used to for the compression of single crystalline compound into a compact without the addition of other substances. The immediate pressure process has points of interest, for example, lesser preparing time and steps, diminished work, lesser process approval, less synthetic solidness issues and so forth. The reason, why CCA has increased extraordinary place in oral medication conveyance framework lies in its straight forwardness and capacity to create round agglomerates in a solitary advance [3,4]. The circular agglomerates acquired can be utilized as spansules or specifically compressible agglomerates. They offer points of interest like magnificent stream characters, uniform size dissemination, and reproducible pressing/filling. Substantial surface territory offered by circles brings about uniform dispersion all through gastro-intestinal tract (GIT) prompting lessening in the restricted lethality [5-7].
In addition, this uniform dissemination may enhance assimilation and bioavailability of medication/s. In light of the low surface zone to-volume proportion contrasted with powder or granules, they can be considered as a brilliant coating substrate [8,9]. Circles indicate change in the remedial characteristics of dose frame because of good dosing and dealing with properties [10]. They are less helpless to dosage dumping [11], and disappointment of a couple of units may not be as important as disappointment of a solitary unit framework. Another critical preferred standpoint of circles lies in that they get minimum influenced by the typical gastric discharging time and consequently medicate conveyance utilizing same is less inclined to physiological factors [12]. It has been accounted for that pellets littler than around 2.4 mm breadth are free from stomach related capacity of the stomach and the end arrangement of the pyloric sphincter to be discharged from the stomach. A greatest pellet breadth of 1.5 mm has been prescribed for an ideal various unit definition [13,14].
A few discoveries have referred to limit measure beneath 1 mm. The impact of both thickness and size of the pellet influences the gastrointestinal travel time [15,16]. The higher thickness of the pellets has delayed the gastric travel time, while the bigger size marginally drawn out the little gut travel time however not the gastric travel time[17].
Advantages[18]
- a. Great stream properties.
b. Uniform size dissemination.
c. The procedure is extremely straightforward
d. Less handling cost which makes the generation financial.
e. Unit tasks are minimal.
f. Single step age of agglomerates.
g. The procedure requires less work - one individual required for whole task.
h. The straightforwardness in the process helps in empowering the maker to go along effectively with CGMP.
i. Crystallo co agglomerates can be utilized as tablet intermediates and for the plan of MUPS.
j. Large surface territory that empowers uniform dispersion of medication through gastro-intestinal tract. This thus helps in diminished poisonous quality, enhanced assimilation and along these lines sufficient bioavailability.
k. The low surface region to volume proportion makes them incredible holding up substrates
l. They have great remedial characteristics because of enhanced dosing and taking care of properties.
m. They are minimum influenced by gastric discharging.
n. Their medication conveyance is less inclined to physiological factors.
o. They indicate less measurements dumping.
p. If the pellet measure is under 2.4 mm distance across, at that point they are free from gastric stomach related capacity and shutting arrangement of pyloric sphincter
Kinetics for spherical crystallization & crystallo-co- agglomeration
a. ProcessInitial works reported that spherical crystallization follows first order or second order kinetics, but detailed work on mechanism of agglomeration have shown that agglomeration process follows first order kinetics.[8] This behaviour is explained by the restricted movement of particles in space due to particle interaction, such as layering agglomerates of fine particles on coarse ones.
Spherical crystallization process has been described by a selective coalescence mechanism. The kinetic equation is,
Log d = C log t + C' (λ) (1.1)
Where, d = diameter of agglomerates (mm)
t = agglomeration time (min)
C' (λ) = function of coalescence time
C = constant
During crystallo-co-agglomeration process, agglomerates were spheronized and compacted. The compaction process of agglomerates was represented by the changes in porosity of agglomerates with agglomeration time. The agglomerates were more easily compacted by increase in agitation speed and amount of bridging liquid, because they increase the sheer force applied to agglomerates as well as enhance the plasticity.
Process Design Studies
In CCA, synchronous crystallization and agglomeration of particles are done in a solitary advance and circular agglomerates are acquired. The framework outline for CCA prescribes utilization of good solvent to solubilise drug, non-solvent to cause precipitation/crystallization of drug/s and the crossing over fluid which basically must be immiscible with non- to shape the fluid extensions between solidified particles and insoluble solids amid the procedure of agglomeration. Once in a while crossing over fluid goes about as a decent soluble also [19]. Till date, two strategies have been created for CCA. Solvent change technique includes synchronous crystallization and agglomeration of at least two medications from a decent soluble and spanning fluid by expansion of a non-dissolvable. The second strategy includes crystallization of medication from a framework containing a decent soluble and crossing over fluid and its synchronous agglomeration with insoluble medication/diluents by expansion of a non-soluble.Determination of both of these techniques requires information of the physicochemical properties of medication and dissolvable framework. Once the technique has been chosen, at that point its preparing should be possible in a vessel portrayed by Morishima et al. [13,20] for SC. The controlled tumult of substance in Morishima vessel creates round agglomerates. The endpoint of the agglomeration procedure can be judged by the span of agglomerates, clearness of supernatant and vaporization of natural solvent/s from the agglomeration framework. Successful outline of the CCA procedure relies upon various variables influencing the procedure of crystallization and agglomeration. Or maybe, it is an exceptionally complex procedure to be examined, getting affected by various definition and process factors. Different components influencing CCA have been described ahead.
Formulation Factors/Variables
a. Diluent selectionThe utilization of diluent has been proposed in CCA for size enlargement of low measurements drugs. Diluent chose must be physico-chemically and physiologically inert, and cheap. Also, it ought to be insoluble in the watery stage to keep away from the misfortunes through the constant/outside phase. Considering wanted qualities, powder has been utilized as a diluent in the improvement of the CCA procedure [21]. By utilizing powder, placebo beads have been set up by Limzerwala. Along these lines, Gadekar and Jadhav have built up the procedure for size enlargement of low dosage bromhexine hydrochloride (BXH) utilizing Talk as diluents [21]. On similar lines, utilization of powder has been made by Pawar in the agglomeration of ibuprofen, a high dose drug [22,21]. No reports on the gastrointestinal disorders caused by Talk upon oral ingestion have been showed up [23]. Adsorption considers have indicated minimum adsorption of cimitedine [24] and bromhexinehydrochloride [25] on Talk. Along these lines, it can be inferred that, claim of Talk as an excipient/diluent in dab/ pellet making gets fortified further. As of late, starch and Na-starch glycolate has been utilized as a part of arrangement of rapidly disintegrating agglomerates of naproxen by the CCA procedure.
b. Solvent System
The solvent system choice for the CCA procedure relies upon solubility and stability of medication/s. Since, dominant parts of medications are soluble in organic solvents and inadequately soluble in water. Utilization of organic solvent (generally nontoxic) has been suggested as a good solvent and additionally bridging fluid and water as an outside/preparing stage (non-solvent). This kind of solvent choice has been recommended because of rare prerequisite of organic solvent [21]. The bridging fluid should complete particular wetting of crystals/solids and frame fluid extensions amid the procedure of agglomeration, and at the same time, it ought to be immiscible with a non-solvent. On the off chance that bridging fluid is utilized as a good solvent, it implies, it performs double part of a good solvent and bridging fluid. The good solvent utilized ought to be unpredictable and immiscible with nonsolvent to maintain a strategic distance from tranquilize misfortune because of co-dissolvability [21,22].
c. Dispersion of internal phase
The internal phase made out of medication solution/suspension with or without diluent and bridging liquid ought to be effectively emulsified/scattered in the outer phase. The procedure can be helped by determination of different appropriating operators/ dispersants. Different hydrophilic polymers and surfactants, for example, polysorbates, polyvinyl pyrrolidone (PVP), and polyvinyl liquor (PVA) have been accounted for to encourage scattering in ideal fixations [19].
d. Polymers
It was discovered that the Crystallo co agglomerates unadulterated drugs have poor compressibility and handling characteristics. This will keep the utilization of direct compressing in tablet making and accordingly falls flat the reason. So different polymers like hydroxy propyl methylcellulose (HPMC), poly ethylene glycol (PEG), ethyl cellulose (EC) and poly vinyl pyruvate (PVP) were used. This enhances the micromeritics mechanical and drug discharge properties of the agglomerates [19].
e. Drug Loading
The degree of drug loading in agglomerate changes the necessity of bridging liquid, good solvent, and non-solvent in CCA. It has been watched that the drug loading pronouncedly affects the general nature of agglomerates. An expansion in drug loading has indicated expanded drug misfortune through the outside stage. On the off chance that the framework has insoluble diluent/excipient, solidified drug gets kept on its surface and creates the miniscular type of drug. The impact of drug content on tablet ability and drug discharge attributes of bromhexineHCl-powder agglomerates arranged by Crystallo-co-agglomeration has been contemplated by Jadhav [26,27]. It has been accounted for that in spite of known poor cohesively of BXH, its part in enhancing rigidity has been set up at higher drug stack in agglomerates. The impact of elasticity in accomplishing broadened drug discharge has likewise been underlined. At long last, it was inferred that the drug content deciding elasticity of smaller is in charge of the accomplishment of expanded drug discharge from minimized.
f. Drug loss to supernatant
The drug loss to supernatant determines the drug entrapment and the overall efficiency of the CCA process. During the agitation process, maximum crystallization and agglomeration of drug/s should be ensured. Attempts have been made to reduce the drug loss by processing the contents at low temperature, pH adjustments, and addition of solubility suppressants to the external phase [19].
g.Yield of the process
The process yield depends on the amount of crystallisation occurred from the good solvent as well as the extend ofagglomeration from the bridging liquid. Thus the selection of solvent system holds an important role in the process yield of Crystallo co agglomeration. The solubilisation of drug is determined by the good solvent and the crystallisation is done by the non solvent. The bridging is an interparticular interaction. Hence for obtaining desirable yield proper selection of solvent system is recommended [28].
Process Variables
a. AgitationThe primary capacity of agitation is emulsification or scattering. The size, shape, sphericity and quality of the agglomerates were influenced by agitation. Rapid fomentation may bring about expanded sphericity and diminished quality of the agglomerates. It was additionally discovered that with the expansion in speed of agitation, it might diminish the time required for the procedure and it decreases the agglomeration [4].
b. Time required for batch processing
The season of agitation chooses the fulfilment of agglomeration. Fragmented agitation prompts deficient blending of different ingredients, in this manner inadequate development of agglomerates. This likewise reduces the evaporation of organic solvents from the response vessel, while overabundance agitation results in fine arrangement [29]. The end purpose of agglomeration assurance is basic in CCA. It can be discovered by judging the clarity of the supernatant, leftover natural solvent and achievement of appropriate agglomerate size [4].
c. Evaluation Techniques Used for the Crystallo co Agglomerates
i. Surface Topography [30]
In surface topography studies, the agglomerates were captured utilizing an optical magnifying lens with camera at its unique amplification. The zone (An) and edge (P) of the agglomerates were gotten from tracings of augmented photomicrographs. This can be utilized to ascertain shape factor (S).
S = P2 actual/ (4nA actual).
Twenty granules per batch can be evaluated.
ii. Differential scanning calorimetry
Differential scanning calorimetry incorporates the estimation of changes that happen when heat to the example while they are subjected to controlled temperature programming [31].
DSC thinks about the thermo tropic conduct of particles. The procedure like crystallization can be watched utilizing DSC. At the point when temperature of an example is expanded step by step the consistency of undefined solids will diminish. At a specific point the atoms may achieve adequate vitality in order to mastermind themselves into gems. This temperature is crystallization temperature (Tc). This procedure of change of a shapeless strong into a crystalline strong is an exothermic procedure and is shown in the thermogram (diagram acquired) as a pinnacle. This guideline is utilized as a part of the investigation of crystallo co agglomerates. Thermograms of medications, polymers and agglomerates are performed utilizing a differential scanning calorimetry. The DSC temperature ought to be adjusted. Precisely measured examples are fixed in an aluminium cauldron. The framework ought to be cleansed with nitrogen gas.
iii.Micromeritics Properties
With a specific end goal to get consistency in tablet weight, the agglomerates must stream and pack easily into the die cavity of the tabletting punching machine.. Along these lines, micromeritics properties are assessed for molecule plan of agglomerates for guide pressure to enhance the stream and pressing properties of pharmaceutical powders. Agglomerates are assessed for flow ability by Angle of repose utilizing the settled fixed funnel free standing cone method. Molecule estimate circulation is considered by sifter investigation. In this agglomerates held on strainers are weighed and the subsequent information is utilized to acquire the mean geometric diameter by plotting the aggregate rate undersize versus the normal size on log average particle size on log probability paper. Qualities for angle of repose = 30 in. dictate free streaming material while angle of repose = 40 demonstrate poor streaming material [3].
iv. Sphericity Determination
Sphericity of the agglomerates is the most imperative attributes and diverse procedures have been utilized to decide it. For satisfactory nature of agglomerates the shape factor ought to be between 1 and 1.2 while 0.6 estimation of shape factor describes great sphericity of agglomerates. The shape factor is dictated by assessing the sum by which the anticipated picture of particles go amiss from a circle and ascertained by methods for the anticipated zone of the agglomerates and its periphery Photomicrographs got by optical magnifying instrument are utilized to figure the region (An) and edge (P) of agglomerates. The molecule shape of both groups is estimated by estimating the shape factor, circularity factor and length-to-width proportion [32].
Shape Factor (P) = P1/2/P'
Where P = 2n (A/7) 1 /2
Circularity Factor (5) = (P) 2 / (12.56*A)
v. Crushing Strength
Crushing strength is assessed to decide mechanical strength since it specifically mirrors the mechanical strength of smaller or tablet. Agglomerates ought to have great mechanical strength on account of expanded in. Trap particle constrain within the agglomerated crystals [33]. Crushing strength of agglomerates is controlled by mercury load cell method. Agglomerates of various bunches are arbitrarily tested and subjected to crushing strength assurance and normal was taken. The logarithmic relationship was set up between crushing strength (CS) and agglomerate size as appeared by the accompanying equation [34]:
log CS=m log D+C
Where m is slope, D is agglomerate diameter and C is intercept calculated by regression analysis of the log D vs. log CS.
vi. Dissolution Studies
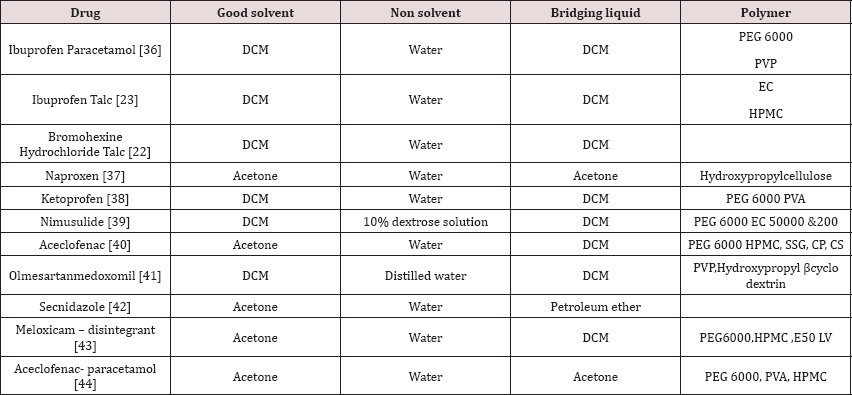
The agglomerate framed by CCA is then subjected to dissolution studies keeping in mind the end goal to understand the pharmacokinetics and in this way bioavailability of the acquired item. U S P suggests dissolution studies for the dissolution studies, which incorporate rotating basket type apparatus, rotating paddle apparatus, reciprocating cylinder apparatus, flow through cell apparatus, and paddle over cell apparatus, cylinder apparatus, and reciprocating plate apparatus. Any of the above gadgets can be utilized for dissolution studies relying on the type of tablet assessedi. e. regardless of whether traditional, controlled release and so on (Table 1)[35-44].
Table 1: Works Held On Cca till this Date.
Conclusion
For more Lupine Publishers Open Access Journals Please visit our website:
http://lupinepublishers.us/
For more Research and Reviews on Healthcare articles Please Click Here:
https://lupinepublishers.com/research-and-reviews-journal/
To Know More About Open Access Publishers Please Click on Lupine Publishers
Follow on Linkedin : https://www.linkedin.com/company/lupinepublishers
Follow on Twitter : https://twitter.com/lupine_online



No comments:
Post a Comment